For many years, there was just coenzyme Q10, also known as ubiquinone. Then there was something new on the market — Ubiquinol. What is this new form? Does it play a different role than coenzyme Q10? What are its unique benefits?
Ubiquinol and coenzyme Q10 are in fact very similar molecules. But the small alteration that makes them different is what gives them their unique role in energy production. That change also makes a big difference in the bioavailability, transportability, and antioxidant activity in the body.† Read on to learn more about all of this.
The difference between Ubiquinol and coenzyme Q10 lies in two electrons (along with the 2 hydrogen atoms, “H,” that come with the electrons). Look at the structures below – see the two lone “Os” (oxygens) of coenzyme Q10? Add the two electrons and the hydrogens and those Os turn into “OHs” (hydroxyl groups), and now you have Ubiquinol. Oh!
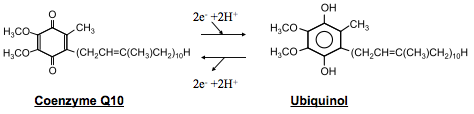
Not only can coenzyme Q10 switch into Ubiquinol, but Ubiquinol can just as easily give up those two electrons and hydrogens, and switch right back into coenzyme Q10. This does in fact happen many times a minute inside the body. When the electrons come on, the molecule is called “reduced,” and when they come off, it is called “oxidized.” This is why Ubiquinol is called “the reduced form of coenzyme Q10.”
So what’s the big deal? Well, this flipping back and forth between the two forms holds the key to the major role of coenzyme Q10/Ubiquinol in the body – producing energy from the food you eat. Does it get more essential? This is much like the way a car burns gas in the engine to provide the energy to keep it moving. In your body, the engines are little machines called mitochondria that are found inside almost all of your cells.
How is food turned into energy? The bonds that hold together a molecule of food, say sugar, contain lots of energy. By breaking those bonds, that energy is released and can be captured and used. The capturing mechanism is like a line of crisis workers who are quickly passing sandbags one to the next to get them to the site of use. Coenzyme Q10/Ubiquinol is like one of these workers, and what it is passing is the released energy, in the form of… electrons! So coenzyme Q10/Ubiquinol spends its day flipping back and forth as it passes electrons along to their final destination, while the energy is put into a usable form.
And you can also see how a deficiency in coenzyme Q10/Ubiquinol would have a great impact on the body’s ability to produce energy.
Now, let’s take a look at some important properties of coenzyme Q10 versus Ubiquinol. As you may know, coenzyme Q10 is not highly absorbable in the body. But Ubiquinol is.1-8 The amount of absorption will vary based on a person’s age and state of health, but in every published comparative study that has been done, Ubiquinol has consistently been much better absorbed than coenzyme Q10.†1-6
We also said there is a difference in transportability. In fact, Ubiquinol is the form that is most preferred by the body for transport in the blood.† In a healthy adult, more than 95% of the total coenzyme Q10 in the blood is in the Ubiquinol form.†7-9 If coenzyme Q10 is ingested, the body quickly transforms it into Ubiquinol. Some people cannot perform that transition very efficiently, and therefore receive very little benefit if they take coenzyme Q10 as opposed to Ubiquinol (look for a future blog on this topic!).
Lastly, Ubiquinol acts as an antioxidant in the body, but coenzyme Q10 does not.† This is due to the two extra electrons Ubiquinol holds.10 What makes a free radical so dangerous is that it is looking to steal electrons… and it will steal them from whatever it comes in contact with – your DNA, cell membranes, cholesterol floating around in your blood. All of these become damaged and/or disease-causing when they lose their electrons! So if the electrons can come from something that doesn’t mind giving them up (like Ubiquinol), then the free radical is neutralized, everyone is happy and no damage occurs.
What’s more, Ubiquinol is one of the few antioxidants that works in the fatty (lipid) parts of the body, such as cell membranes and low density lipoprotein (LDL) cholesterol.10 But one of the most important places it acts as an antioxidant is in the mitochondria themselves. This is because the mitochondria are factories for free radicals. Just as in our car analogy the engine produces exhaust (which is full of free radicals!) the mitochondria has its form of free radical-laden exhaust. Therefore, Ubiquinol has a big job protecting the mitochondria and their lipid membranes from attack.10-12 Ubiquinol also supports the whole antioxidant network in the body by recycling vitamin E, the other lipid-soluble antioxidant, and vitamin C.11,12
Now you are an expert on the differences between coenzyme Q10 and Ubiquinol. They are both critical for energy-production, but there are big differences when you look at bioavailability and transportability. In addition, only Ubiquinol acts as a rare lipid-soluble antioxidant.
For more information on Ubiquinol, visit Ubiquinol.org
† This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent disease.
Always consult with your physician before taking any supplements alone or in conjunction with pharmaceutical drugs.
Citations
- Miles MV, Horn P, Milesc L, Tanga P, Steele P, DeGrauwa T. Bioequivalence of coenzyme Q10 from over-the-counter supplements. Nutr Res. 2002:22(8):919-929.
- Bhagavan HN, Chopra RK. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion. 2007 Jun;7 Suppl:S78-88.
- Evans M, Baisley J, Barss S, Guthrie N. A randomized, double-blind trial on the bioavailability of two CoQ10 formulations. Journal of Functional Foods. 2009. 1: 65-73.
- Mohr D, Bowry VW, Stocker R. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim Biophys Acta. 1992 Jun 26;1126(3):247-54.
- Shoko D, Fujii K, Kurihara T. The effect of the reduced form of coenzyme Q10 (Ubiquinol, Kaneka QH™ ) on QOL improvement in the elderly. J Clin Therap Med 2008; 24:233-238.
- Schmelzer C, Niklowitz P, Okun JG, Haas D, Menke T, Döring F. Ubiquinol-induced gene expression signatures are translated into altered parameters of erythropoiesis and reduced low density lipoprotein cholesterol levels in humans. IUBMB Life. 2011 Jan;63(1):42-8.
- Hosoe K, Kitano M, Kishida H, Kubo H, Fujii K, Kitahara M. Study on safety and bioavailability of ubiquinol (Kaneka QH) after single and 4-week multiple oral administration to healthy volunteers. Regul Toxicol Pharmacol. 2007 Feb;47(1):19-28.
- Tang PH, Miles MV, DeGrauw A, Hershey A, Pesce A. HPLC analysis of reduced and oxidized coenzyme Q(10) in human plasma. Clin Chem. 2001 Feb;47(2):256-65.
- Yamashita S, Yamamoto Y. Simultaneous detection of ubiquinol and ubiquinone in human plasma as a marker of oxidative stress. Anal Biochem. 1997 Jul 15;250(1):66-73.
- Frei B, Kim MC, Ames BN. Ubiquinol-10 is an effective lipid-soluble antioxidant at physiological concentrations. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4879-83.
- Littarru GP, Tiano L. Bioenergetic and antioxidant properties of coenzyme Q10: recent developments. Mol Biotechnol 2007;37(1):31-37.
- Ernster L, Forsmark-Andrée P. Ubiquinol: an endogenous antioxidant in aerobic organisms. Clin Investig. 1993;71(8 Suppl):S60-5.
- Hargreaves IP, Duncan AJ, Heales SJ, Land JM. The effect of HMG-CoA reductase inhibitors on coenzyme Q10: possible biochemical/clinical implications. Drug Saf. 2005;28(8):659-76.
